Mitochondria Transplantation Therapy – It’s Farther Along Than You Thi…

-
- 첨부파일 : bg.png (203.5K) - 다운로드
-
1656회 연결
-
2537회 연결
- - 짧은주소 : http://mito.cha.ac.kr/mito/bbs/?t=8H
본문
Mitochondria Transplantation Therapy – It’s Farther Along Than You Think
“The future is already here – it’s just not evenly distributed.” -William Gibson
The whole idea sounds a little fanciful, almost like something from Star Trek. However, it may surprise you that mitochondrial transplantation (sometimes called “mitochondrial augmentation”) therapies have already been tried in humans and that there’s a firm rationale for this biomedical technology based on two decades of basic research in cells and in vivo. Although nature came up with the “idea” first (a story blogged by RoosterBio that was 2 billion years in the making), 1 converging trends in cell manufacturing, synthetic biology, and bioinformatics are today transforming engineered organelle therapies from mere theory into practice. Toward this end, mesenchymal stromal/stem cells (hMSCs) are sure to play a pivotal role.
A Picture Speaks 1,000 Words?
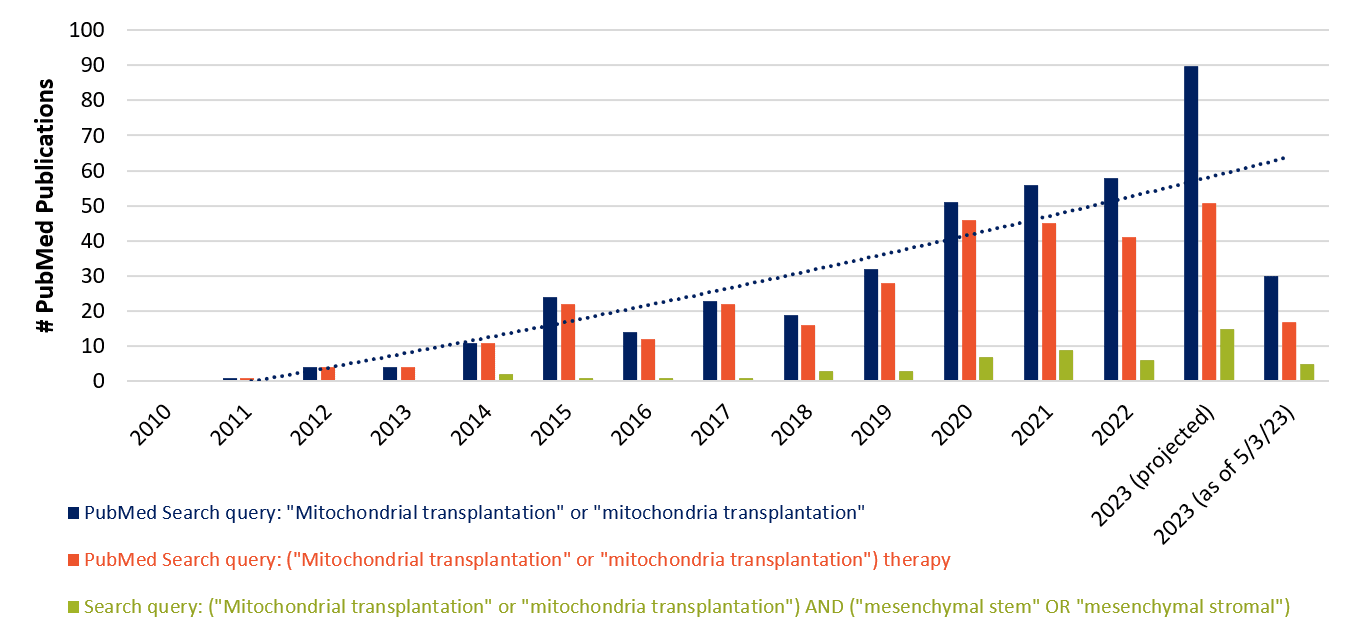
Figure 1. Above, publications related to mitochondrial transplantation in PubMed are accelerating year to year according to counts of targeted queries. A substantial fraction of these publications also involves the term “therapy” or “mesenchymal stem” cells.
Does simple query language to PubMed predict a medical revolution in the making? The terminology related to mitochondrial transplantation was seldom heard until the mid-2010s, but now it’s trending upward year-to-year. This trend would seem to be accelerating, if 2023 publication counts via mid-Spring can be sustained through December (Figure 1). Moreover, mitochondrial transplantation is frequently found together with the word “therapy”, indicating closely aligned ideation between this tech and clinical translation. Finally, a significant fraction of these publications also involves mesenchymal stromal/stem cells.
Mitochondria “donated” from stem cells and/or hMSCs seem to play a physiologic role in tissue maintenance and repair. We learned in 2006 that mitochondria-negative, A549 ρ° cells receive transferred “mitos” via MSCs in co-culture, thereby rescuing their capacity for aerobic respiration. 2 Subsequent observations posited that such cell-to-cell transfer might occur through tunneling nanotubes, 2, 3 larger vesicles, 4 cell fusions, 5 or cell-cell contacts. 6 Incidentally, it’s also reported that cells and tissues can engulf free-floating, “naked” mitochondria. 7 Injected doses of these may elicit cardioprotection in rabbit models of ischemia of the “widow maker” left-anterior descending artery, inspiring research towards a novel biologics drug system. One of several excellent non-paywalled reviews on this topic is from Gomzikova, James, and Rizvanov (2021). 8 Although there is still much to be learned, the homeostatic features of “hMSC-mitos” may be one reason why investigators are prospecting mesenchymal stromal cells as parent bioproducers for their therapeutic platforms.
Investigators Turn to MSCs for Mito-Sourcing
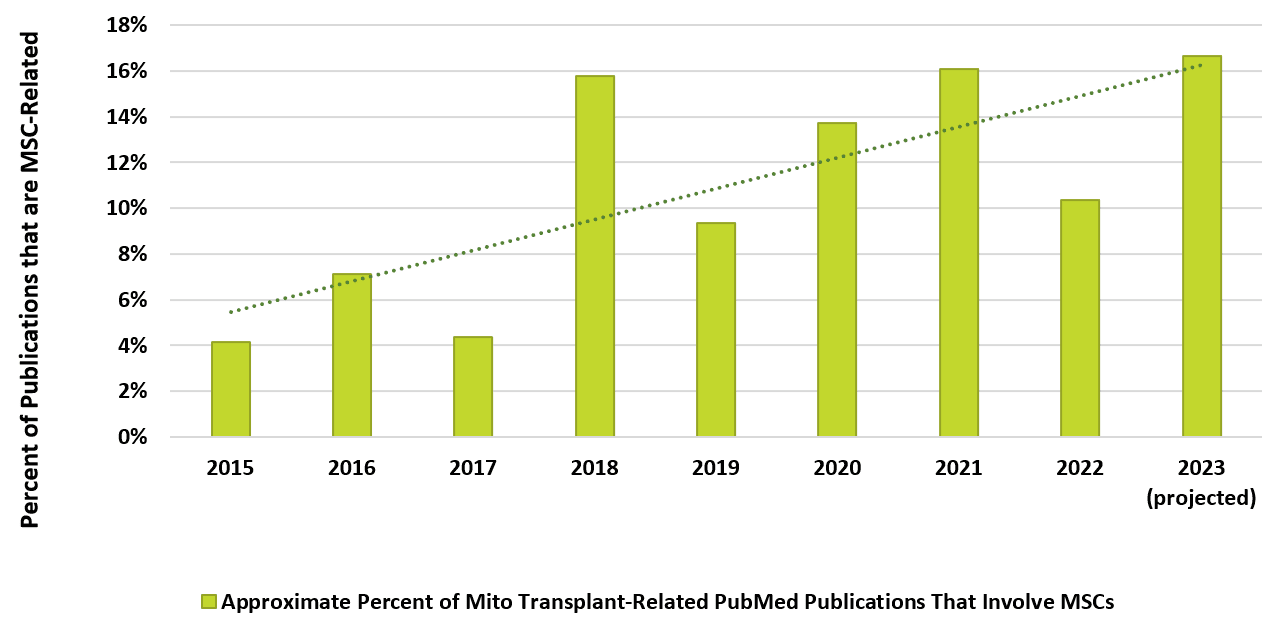
Figure 2. Above, MSCs account for an increasing fraction of publications related to mitochondrial transplantation.
Given the safe track record from over 1500 worldwide listed human clinical trials involving MSCs since 2011 9 and 10 MSC cell therapy products marketed beyond the USA, 10 it’s no surprise that aspiring clinical product developers are using MSCs as a source for transplantable, therapeutic mitochondria. The fraction of publications related to mito transplantation that specifically involve MSCs has increased at least threefold since 2015 to over 15% (Figure 2). This pattern parallels the preponderance of worldwide clinical trials involving extracellular vesicle (EVs) and/or exosome material isolated from MSCs—now over 60%. 11
Human Trials Now in Full Swing
Transplanted mitochondria have been used or proposed for experimental human therapies targeted towards infertility, 12, 13, 14 life-threatening ischemic heart damage, 15, 16, 17 mitochondrial deletion syndromes, 18 lung injury, 6 brain damage, 19, 20, 17 sepsis, 21 and other pathologies.22 This new therapeutic modality has even attracted coverage in major media outlets. 16, 23 A list of seven human trials mined from clinicaltrials.gov spans a variety of indications from cardiac ischemia-reperfusion injury in children to Parkinson’s (see Table 1). Four of these (~57%) use mitochondria derived from hMSCs (shown by orange NCT Number).
| NCT Number | Title | First Posted | Phases | Sponsor / Collaborators | Status (3/22/23) | Conditions | Interventions |
| NCT02851758 | Transplantation of Autologously Derived Mitochondria Following Ischemia | 2-Aug-16 | Not Applicable | Boston Children’s Hospital | Recruiting | Extracorporeal Membrane Oxygenation Complication | Other: autologous mitochondria transplantation |
| NCT03384420 | A Study to Evaluate the Safety and Therapeutic Effects of Transplantation of MNV-BM-BLD in Pediatric Patients With Pearson Syndrome | 27-Dec-17 | Phase 1|Phase 2 | Minovia Therapeutics Ltd. | Completed | Mitochondrial Diseases|Pearson Syndrome | Biological: CD34+ cells enriched with MNV-BLD |
| NCT03639506 | Clinical Application of Autologous Mitochondria Transplantation for Improving Oocyte Quality. | 21-Aug-18 | Not Applicable | Sun Yat-sen University | Unknown status | Repetition Failure | Procedure: autologous mitochondria transplantation|Combination Product: autologous mitochondria from bone marrow mesenchymal stem cells into oocyte as well as intracytoplasmic sperm injection (ICSI)|Drug: intracytoplasmic sperm injection (ICSI) |
| NCT04976140 | Allogeneic Mitochondria (PN-101) Transplantation for Refractory Polymyositis or Dermatomyositis | 26-Jul-21 | Phase 1|Phase 2 | Paean Biotechnology Inc. | Enrolling by invitation | Polymyositis|Dermatomyositis | Biological: PN-101 |
| NCT04998357 | Autologous Mitochondrial Transplant for Cerebral Ischemia | 10-Aug-21 | Phase 1 | University of Washington | Recruiting | Cerebral Ischemia | Other: Endovascular autologous mitochondrial transplantation |
| NCT05094011 | Evaluating Safety, Tolerability, and Efficacy of Autologous MitoCell Transplantation in Subjects With Idiopathic Parkinson’s Disease | 26-Oct-21 | Phase 1 | Taiwan Mitochondrion Applied Technology Co., Ltd. | Not yet recruiting | Idiopathic Parkinson’s Disease | Biological: Adipose-Derived Mesenchymal Stem Cells |
| NCT05669144 | Co-transplantation of Mesenchymal Stem Cell Derived Exosomes and Autologous Mitochondria for Patients Candidate for CABG Surgery | 30-Dec-22 | Phase 1|Phase 2 | Tehran University of Medical Sciences | Recruiting | Myocardial Infarction|Myocardial Ischemia|Myocardial Stunning | Biological: mitochondria and MSC-derived exosomes |
Table 1. Above, seven interventional clinical trials using mitochondria used as materials for organelle transplantation, identified from a recent query to clinicaltrials.gov. Orange NCT # color, mitochondria material from hMSCs.
Mito Companies Hatching for Fully Fledged Clinical Translation
Interest in mitochondrial transplantation is not merely academic. Cursory searches around the web turned up at least seven companies related to some variation of the technology tied to aspirations for the clinic (Table 2). Of these, three appear to have entered or are soon approaching human trials (Minovia, Paean Bio, and Taiwan Mitochondrion Applied Technology). Several others (not shown) are dedicated to enabling mitochondria-directed tools and instruments that could play important supporting roles with advanced therapies in humans.
| Name | URL | Description |
| Mitrix Bio | mitrix.bio | Based in California, Mitrix is working on “large scale Mitochondrial Transfusion” to combat neurodegenerative diseases and juvenile mitochondrial mutation diseases. “In the Mitrix process, healthy mitochondria are grown in bioreactors and transfused into the patient to bolster energy production and regenerate aged or dysfunctional tissues. The Mitrix team are recognized leaders in the field, working at research facilities around the world.” |
| Minovia | minoviatx.com | Haifa’s Minovia Therapeutics “is a clinical-stage international biotechnology company, established in hopes to bring life changing therapies to patients with Mitochondrial diseases, as fast as responsible. Following compassionate use cases performed in Sheba Medical hospital in Israel, using our proprietary Mitochondrial Augmentation Therapy, Minovia is now conducting the first of its kind clinical trial for Pearson syndrome, an ultra-rare pediatric disease.” |
| cellvie | cellvie.bio | cellvie operates out of Houston and Zurich. “Employing proprietary preparation and delivery techniques, cellvie is transplanting mitochondria directly into compromised cells. The organelles penetrate the cell walls through endocytosis and merge with the mitochondrial network, to contribute to the cells’ energy metabolism.” |
| PAEAN Biotechnology Inc | www.paeanbio.com | Korea’s Paean Bio uses mitochondria from hMSCs to manufacture PN-101, its flagship clinical product that is aimed at inflammatory diseases such as polymyositis/dermatomyositis, hearing loss, ARDS, NASH, and other conditions. Meanwhile, its “Mitoceutical” technology is developing scFv-functionalized mitochondria as a targeted delivery system for anti-cancer molecules. |
| LUCA Science | www.luca-science.com | Japan’s LUCA Science has developed a novel method to isolate, engineer, and deliver proprietary functional mitochondria. These medicinal organelles can then be stored and delivered as a biopharmaceutical agent. |
| MitoSense | www.mitosenseinc.com | Virginia’s Mitosense discovered and developed Mitochondria Organelle Transplantation (MOT™) technology originally for cancer (PMID: 23080556). Now, the proof of concept for this cellular biotherapy platform is being adapted towards acute TBI and neurodegenerative diseases such as ALS, Parkinson’s and Alzheimer’s. |
| Taiwan Mitochondrion Applied Technology Co. | en.mitobiomed.com | TMAT has developed Mitochondrial Reconstruction Technology (MRT), which extracts high-activity and high-quality healthy mitochondria from cells, reconstructing and repairing mitochondria in damaged cells “to restore mitochondrial function, enhance cell activity, and rejuvenate cells to youth.” |
Table 2. Above, brief descriptions and links to seven companies in operation, with mitochondrial transplantation technology platforms and intentions to use mitochondria materials for organelle transplantation, identified from a brief internet query. At least three are working directly with hMSCs.
MSC-Mitos to Power the Future’s Nano-Engineered Medicine
It’s difficult imagining a disease that couldn’t be modulated by revving up the bioenergetics of target cells with transplanted and intact mitochondria. These “nano-cells” with ancient origins via Precambrian purple bacteria have genomes of their own, of course, that might be just as engineerable as their host cells. Capsid-engineered AAV can directly enter mitochondria to model a Leber’s hereditary optic neuropathy (LHON) 24 in mice by persistent, intra-organelle expression of the pathological mutant via the mitochondrial heavy strand promoter. In turn, an AAV that expresses the wild-type replacement gene (ND4) allotopically can correct the mice’s visual impairment and is the basis for ongoing human trials to correct LHON. 25 The human mitochondrial genome can also be edited with targeted nucleases such TALENs, 26 although efficient methods based on CRISPR-Cas9—requiring intra-organelle co-transport of gRNAs—would need to overcome a few technical hurdles. 27, 28 Taken together, these findings (and many others) inform us of several key points:
- Mitochondria can be transplanted from one cell to another, either vesicle-bound or “naked,” transferring therapeutic effects into diseased cells, tissues, and organs.
- Mitochondria can also be genetically engineered and edited—either directly via the mitochondrial genome—or indirectly through proteins via nuclear expressed DNA transported into the organelle; thus, “mitos” might be converted into a viable “cytosolic” genetic medicine platform.
- Mitochondria surfaces can be chemically derivatized and/or lipid encapsulated to assist in tissue-selective targeting and uptake into zones of disease.
- Mitochondria transplantation therapy has already been attempted in humans, to aid in emergency heart surgeries and even in the (albeit controversial) conception of so-called “three parent children.”
- Producer cells (such as hMSCs) could be mass expanded under cGMP culture & harvesting conditions to yield hundreds to thousands of mitochondria doses.
So, as the cyberpunk author William Gibson is quoted, the “future is here.” RoosterBio stands ready to “more evenly distribute” and accelerate this promising new future that’s gearing up for more breakthroughs within the next decade. We and others will be there to help democratize the innovative “mito-scape” with our comprehensive system of hMSC and EV/exosome-related products, high-performance culture and collection media, genetic engineering media, and bioprocess services and expertise.
References
- Carson, J. From Old Adversaries to RegenMed Housemates: The 2-Billion-Year Story of Mitochondria Transfer via MSCs. https://www.roosterbio.com/blog/from-old-adversaries-to-regenmed-housemates-the-2-billion-year-story-of-mitochondria-transfer-via-mscs/.
- Spees, J. L.; Olson, S. D.; Whitney, M. J.; Prockop, D. J., Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A 2006, 103 (5), 1283-8. 10.1073/pnas.0510511103
- Plotnikov, E. Y.; Khryapenkova, T. G.; Vasileva, A. K.; Marey, M. V.; Galkina, S. I.; Isaev, N. K.; Sheval, E. V.; Polyakov, V. Y.; Sukhikh, G. T.; Zorov, D. B., Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. J Cell Mol Med 2008, 12 (5A), 1622-31. 10.1111/j.1582-4934.2007.00205.x
- Phinney, D. G.; Di Giuseppe, M.; Njah, J.; Sala, E.; Shiva, S.; St Croix, C. M.; Stolz, D. B.; Watkins, S. C.; Di, Y. P.; Leikauf, G. D.; Kolls, J.; Riches, D. W.; Deiuliis, G.; Kaminski, N.; Boregowda, S. V.; McKenna, D. H.; Ortiz, L. A., Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun 2015, 6, 8472. 10.1038/ncomms9472
- Acquistapace, A.; Bru, T.; Lesault, P. F.; Figeac, F.; Coudert, A. E.; le Coz, O.; Christov, C.; Baudin, X.; Auber, F.; Yiou, R.; Dubois-Rande, J. L.; Rodriguez, A. M., Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells 2011, 29 (5), 812-24. 10.1002/stem.632
- Islam, M. N.; Das, S. R.; Emin, M. T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D. J.; Quadri, S. K.; Bhattacharya, S.; Bhattacharya, J., Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 2012, 18 (5), 759-65. 10.1038/nm.2736
- Masuzawa, A.; Black, K. M.; Pacak, C. A.; Ericsson, M.; Barnett, R. J.; Drumm, C.; Seth, P.; Bloch, D. B.; Levitsky, S.; Cowan, D. B.; McCully, J. D., Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 2013, 304 (7), H966-82. 10.1152/ajpheart.00883.2012
- Gomzikova, M. O.; James, V.; Rizvanov, A. A., Mitochondria Donation by Mesenchymal Stem Cells: Current Understanding and Mitochondria Transplantation Strategies. Front Cell Dev Biol 2021, 9, 653322. 10.3389/fcell.2021.653322
- MSC Clinical Trials Data from CellTrials.org. https://celltrials.org/public-cells-data/msc-trials-2011-2020/65.
- Hildreth, C. MSC Therapies: Globally Approved Mesenchymal Stem Cell Therapeutics. https://bioinformant.com/msc-therapies/.
- celltrials.org Exosome Clinical Trials Data from CellTrials.org. https://celltrials.org/public-cells-data/subscribe-to-exosome-clinical-trials/80.
- Woods, D. C.; Tilly, J. L., Autologous Germline Mitochondrial Energy Transfer (AUGMENT) in Human Assisted Reproduction. Semin Reprod Med 2015, 33 (6), 410-21. 10.1055/s-0035-1567826
- Woods, D. C.; Tilly, J. L., Revisiting Claims of the Continued Absence of Functional Germline Stem Cells in Adult Ovaries. Stem Cells 2023, 41 (2), 200-204. 10.1093/stmcls/sxac083
- Yoshihara, M.; Wagner, M.; Damdimopoulos, A.; Zhao, C.; Petropoulos, S.; Katayama, S.; Kere, J.; Lanner, F.; Damdimopoulou, P., In Reply: Revisiting Claims of the Continued Absence of Functional Germline Stem Cells in Adult Ovaries. Stem Cells 2023, 41 (2), 205-206. 10.1093/stmcls/sxac084
- Emani, S. M.; Piekarski, B. L.; Harrild, D.; Del Nido, P. J.; McCully, J. D., Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J Thorac Cardiovasc Surg 2017, 154 (1), 286-289. 10.1016/j.jtcvs.2017.02.018
- Kolata, G. Dying organs restored to life in novel experiments. https://www.nytimes.com/2018/07/10/health/mitochondria-transplant-heart-attack.html.
- Hayashida, K.; Takegawa, R.; Endo, Y.; Yin, T.; Choudhary, R. C.; Aoki, T.; Nishikimi, M.; Murao, A.; Nakamura, E.; Shoaib, M.; Kuschner, C.; Miyara, S. J.; Kim, J.; Shinozaki, K.; Wang, P.; Becker, L. B., Exogenous mitochondrial transplantation improves survival and neurological outcomes after resuscitation from cardiac arrest. BMC Med 2023, 21 (1), 56. 10.1186/s12916-023-02759-0
- Jacoby, E.; Bar-Yosef, O.; Gruber, N.; Lahav, E.; Varda-Bloom, N.; Bolkier, Y.; Bar, D.; Blumkin, M. B.; Barak, S.; Eisenstein, E.; Ahonniska-Assa, J.; Silberg, T.; Krasovsky, T.; Bar, O.; Erez, N.; Bielorai, B.; Golan, H.; Dekel, B.; Besser, M. J.; Pozner, G.; Khoury, H.; Jacobs, A.; Campbell, J.; Herskovitz, E.; Sher, N.; Yivgi-Ohana, N.; Anikster, Y.; Toren, A., Mitochondrial augmentation of hematopoietic stem cells in children with single large-scale mitochondrial DNA deletion syndromes. Sci Transl Med 2022, 14 (676), eabo3724. 10.1126/scitranslmed.abo3724
- Alexander, J. F.; Seua, A. V.; Arroyo, L. D.; Ray, P. R.; Wangzhou, A.; Heibeta-Luckemann, L.; Schedlowski, M.; Price, T. J.; Kavelaars, A.; Heijnen, C. J., Nasal administration of mitochondria reverses chemotherapy-induced cognitive deficits. Theranostics 2021, 11 (7), 3109-3130. 10.7150/thno.53474
- Hayakawa, K.; Chan, S. J.; Mandeville, E. T.; Park, J. H.; Bruzzese, M.; Montaner, J.; Arai, K.; Rosell, A.; Lo, E. H., Protective Effects of Endothelial Progenitor Cell-Derived Extracellular Mitochondria in Brain Endothelium. Stem Cells 2018, 36 (9), 1404-1410. 10.1002/stem.2856
- Yu, S. H.; Kim, S.; Kim, Y.; Lee, S. E.; Park, J. H.; Cho, G.; Ha, J. C.; Jung, H.; Lim, S. M.; Han, K.; Lee, H. K.; Kang, Y. C.; Kim, C. H., Human umbilical cord mesenchymal stem cell-derived mitochondria (PN-101) attenuate LPS-induced inflammatory responses by inhibiting NFkappaB signaling pathway. BMB Rep 2022, 55 (3), 136-141. 10.5483/BMBRep.2022.55.3.083
- D’Amato, M.; Morra, F.; Di Meo, I.; Tiranti,









댓글목록 0