2021년 08월 Ex vivo expanded allogeneic natural killer cells have potent cytolytic activity against cancer cells through different receptor-ligand interactions
첨부파일
-
 Jung_et_al-2021-Journal_of_Experimental__Clinical_Cancer_Research.pdf
(3.0M)
9회 다운로드
DATE : 2021-10-26 09:43:53
Jung_et_al-2021-Journal_of_Experimental__Clinical_Cancer_Research.pdf
(3.0M)
9회 다운로드
DATE : 2021-10-26 09:43:53
관련링크
본문
Background
Recently, allogeneic natural killer (NK) cells have gained considerable attention as promising immunotherapeutic tools due to their unique biological functions and characteristics. Although many NK expansion strategies have been reported previously, a deeper understanding of cryopreserved allogeneic NK cells is needed for specific therapeutic approaches.
Methods
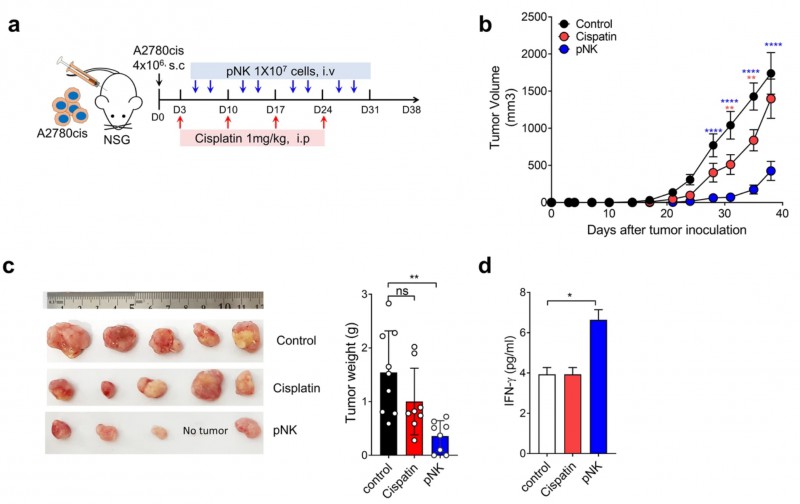
We isolated allogeneic primary natural killer (pNK) cells from healthy donors, and we expanded them ex vivo using a GMP-compliant method without any feeder to generate large volumes of therapeutic pNK cells and cryopreserved stocks. After validation for high purity and activating phenotypes, we performed RNA sequencing of the expanded and cryopreserved pNK cells. pNK cells were used against various cancer cell lines in 7-AAD/CFSE cytotoxicity assay. For in vivo efficacy study, NSG mice bearing subcutaneous cisplatin-resistant A2780cis xenografts were treated with our pNK cells or cisplatin. Antitumor efficacy was assessed by measuring tumor volume and weight.
Results: Compared to the pNK cells before expansion, pNK cells after expansion showed 2,855 upregulated genes, including genes related to NK cell activation, cytotoxicity, chemokines, anti-apoptosis, and proliferation. Additionally, the pNK cells showed potent cytolytic activity against various cancer cell lines. Interestingly, the cryopreserved pNK cells showed a marked increase in NKp44 (1064-fold), CD40L (12,018-fold), and CCR5 (49-fold), suggesting their potent effectiveness against tumors expressing high levels of NKp44 ligand, CD40, or CCL5. The activating pNK cells produced in the study did not express the programmed cell death protein 1, and thus, they were highly cytotoxic against cancer cells highly expressing programmed death-ligand 1 (PD-L1). We also demonstrated the in vitro and in vivo efficacies of pNK cells against cisplatin-resistant A2780cis ovarian cancer cells having a high PD-L1 expression and low HLA-C1 and -C2 expression.
Conclusions
Taken together, our study provides the first comprehensive genome wide analysis of ex vivo-expanded cryopreserved pNK cells. It also indicates the potential use of expanded and cryopreserved pNK cells as a highly promising immunotherapy for anti-cancer drug resistant patients.
Recently, allogeneic natural killer (NK) cells have gained considerable attention as promising immunotherapeutic tools due to their unique biological functions and characteristics. Although many NK expansion strategies have been reported previously, a deeper understanding of cryopreserved allogeneic NK cells is needed for specific therapeutic approaches.
Methods
We isolated allogeneic primary natural killer (pNK) cells from healthy donors, and we expanded them ex vivo using a GMP-compliant method without any feeder to generate large volumes of therapeutic pNK cells and cryopreserved stocks. After validation for high purity and activating phenotypes, we performed RNA sequencing of the expanded and cryopreserved pNK cells. pNK cells were used against various cancer cell lines in 7-AAD/CFSE cytotoxicity assay. For in vivo efficacy study, NSG mice bearing subcutaneous cisplatin-resistant A2780cis xenografts were treated with our pNK cells or cisplatin. Antitumor efficacy was assessed by measuring tumor volume and weight.
Results: Compared to the pNK cells before expansion, pNK cells after expansion showed 2,855 upregulated genes, including genes related to NK cell activation, cytotoxicity, chemokines, anti-apoptosis, and proliferation. Additionally, the pNK cells showed potent cytolytic activity against various cancer cell lines. Interestingly, the cryopreserved pNK cells showed a marked increase in NKp44 (1064-fold), CD40L (12,018-fold), and CCR5 (49-fold), suggesting their potent effectiveness against tumors expressing high levels of NKp44 ligand, CD40, or CCL5. The activating pNK cells produced in the study did not express the programmed cell death protein 1, and thus, they were highly cytotoxic against cancer cells highly expressing programmed death-ligand 1 (PD-L1). We also demonstrated the in vitro and in vivo efficacies of pNK cells against cisplatin-resistant A2780cis ovarian cancer cells having a high PD-L1 expression and low HLA-C1 and -C2 expression.
Conclusions
Taken together, our study provides the first comprehensive genome wide analysis of ex vivo-expanded cryopreserved pNK cells. It also indicates the potential use of expanded and cryopreserved pNK cells as a highly promising immunotherapy for anti-cancer drug resistant patients.